What Can Be Used To Repair Mrna
Abstruse
Many surveillance and repair mechanisms be to maintain the integrity of our genome. All of the pathways described to date are controlled exclusively by proteins, which through their enzymatic activities identify breaks, propagate the damage signal, recruit further protein factors and ultimately resolve the break with petty to no loss of genetic information. RNA is known to have an integral role in many cellular pathways, simply, until very recently, was not considered to accept part in the Deoxyribonucleic acid repair process. Several reports demonstrated a conserved critical role for RNA-processing enzymes and RNA molecules in Dna repair, but the biogenesis of these damage-related RNAs and their mechanisms of action remain unknown. We will explore how these new findings challenge the idea of proteins being the sole participants in the response to DNA damage and reveal a new and heady aspect of both DNA repair and RNA biology.
Facts
-
The miRNA biogenesis machinery has a role in Deoxyribonucleic acid harm repair outside of approved miRNA-mediated translational repression.
-
RNA molecules accept been observed in the proximity of DNA breaks and take been implicated in the Dna repair response.
-
These phenomena have been observed in many species, indicating an evolutionarily conserved mechanism.
Open Questions
-
What is the precise part of the RNA-processing enzymes in Deoxyribonucleic acid repair?
-
Practise modest RNAs have a straight mechanistic function in Deoxyribonucleic acid repair, or do they serve equally a past-product of a unlike RNA species?
-
Is transcription induced locally at sites of DNA damage? Are proximal fallow promoter elements involved, or is an open up-ended intermission sufficient for polymerase recruitment?
-
Tin can these results be replicated exterior of integrated exogenous reporter systems?
An Unlikely Match: RNA Biogenesis Machinery Meets Dna Repair
Our Deoxyribonucleic acid is constantly exposed to various environmental and chemical agents, including ionising radiations (IR) from cosmic radiation, ultraviolet (UV) lite from the sun or fifty-fifty nucleophilic attack induced by chemical compounds in food.1 In fact, Deoxyribonucleic acid impairment is intrinsic to the process of life: it is inevitable during replication and essential during meiotic recombination. As well, controlled DNA breaks by topoisomerase occur to facilitate the resolution of supercoiled chromatin structures. Complex mechanisms take evolved to counteract the variety and quantity of DNA impairment encountered daily.
By and large, DNA damage response (DDR) involves a complex signalling cascade initiated past one of iii PI3K-similar kinases: ATM, ATR or Dna-PK. They serve to facilitate chromatin modification and remodelling, allowing access to and acting as scaffolds for proteins involved in repair, also as propagating the harm point.1 Many of these recruited factors are involved in a binary conclusion-making process (see Figure i). The repair of double-strand breaks (DSBs) is resolved past two distinct mechanisms: error-free homologous recombination (HR) or fault-prone non-homologous terminate-joining (NHEJ).1, two The choice of which mechanism is used can depend on chromosomal context and is cell bike stage dependent;three Hour is favoured when sister chromatids are available in G2 phase, whereas NHEJ is favoured over 60 minutes in the G1 phase of the cell wheel, and in resting or terminally differentiated cells.2, 4 Commitment to the Hour pathway is facilitated past the eviction of key repair proteins, such as 53BP1, from the damage site.2 This is followed past the recruitment of pro-HR proteins, such every bit BRCA1, FancD2 and CtIP, leading to the resection of Deoxyribonucleic acid around break sites and the search of homologous chromatids for template-mediated repair.five Conversely, stabilisation of 53BP1 at break sites by PTIP and Rif1 blocks resection, causing NHEJ to occur.6, 7
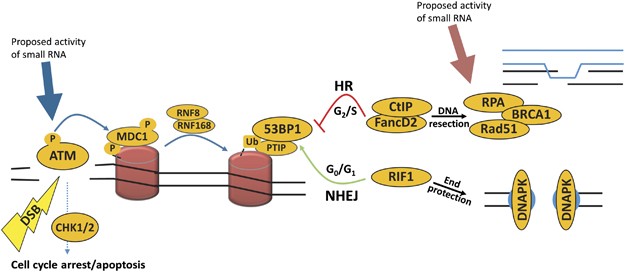
A schematic of the DNA repair pathway. The germination of a DSB induces the phosphorylation of ATM, which contributes to the activation of the DNA repair pathway and cell cycle abort. A series of molecular signalling events lead to the deployment of ubiquitylation (Ub) marks on the histones (red cylinders) in the proximity of DNA breaks, facilitated by RNF8 and RNF168. The recruitment of 53BP1 marks the key crossroad of DSB repair (DSBR) pathway, which branches out into error-gratuitous HR or relatively error-prone NHEJ. Pocket-sized RNAs accept been proposed to part at two singled-out steps in DSBR. Francia et al. 11 suggested that it affects early signal propagation through ATM phosphorylation (blueish arrow), while Gao et al. 8 proposed that it only affects the Hr sub-pathway via modulation of Rad51 binding (red arrow)
Traditionally, it has been thought that DNA repair involves simply enzymatic reactions carried out by proteins that facilitate repair and propagate signalling events. Interestingly, a number of reports have now implicated RNA in DDR.8, 9, 10 These have largely full-bodied on the interest of the small RNA biogenesis enzymes (outlined in Effigy 2) and have identified a novel species of small RNA, which appears to be derived from the vicinity of the DSB. The interest of an RNA species in DDR is well-conserved evolutionarily with observations in fungi, yeast, plant, Drosophila and homo cells.nine, 11, 12, 13, fourteen, 15 The get-go description came from the filamentous fungus Due north. crassa, where interplay between non-approved small-scale RNAs and the DDR was reported. Chemically induced replication stresses in N. crassa resulted in the production of modest RNAs originating mostly from highly transcribed and repetitive ribosomal loci. This event was dependent on the presence of the fungal orthologue of Argonaute protein and an RNA-dependent RNA polymerase.14 Although required for proficient DNA repair, these pocket-sized RNAs appeared to exist produced from the deposition of longer RNA species.14 The authors proposed that aberrant transcripts ('aRNA') transcribed as a result of Dna harm are amplified by RNA-dependent RNA polymerases (RdRPs) and processed into pocket-sized RNA (termed quelling-induced RNA, qiRNA). These qiRNAs and so act to degrade aRNA, in a style similar to the siRNA amplification bike.12, 16, 17 These aRNAs are transcribed from repetitive loci, such equally the ribosomal DNA locus, and are refractory to RNA polymerase inhibitors.14 Interestingly, the production of aRNAs is dependent on the presence of replicating protein A, a known component of the HR repair pathway.xviii How such a machinery could aid in repair of a break itself is unclear. Even so, one tin imagine the suppression of these abnormal transcripts past qiRNA serves to complement nonsense-mediated decay to limit any possible translation of abnormal transcripts.
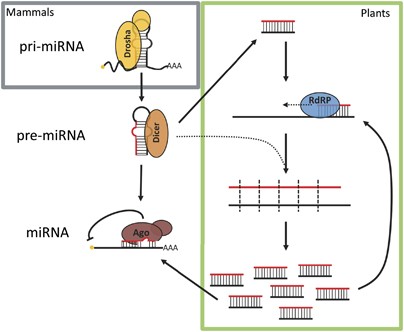
Outline of the microRNA biogenesis pathway in humans, and how plants utilise RdRPs to amplify these. The miRNA gene is transcribed by RNA polymerase Ii and typically capped and polyadenylated. This master miRNA (pri-miRNA) contains the hairpin structure that is recognised and broken past Drosha, as part of the Microprocessor complex. The stem loop is and then further trimmed by Dicer forming the pre-miRNA. In the canonical miRNA pathway, a unmarried strand of the small RNA duplex is loaded into an Argonaute protein (Ago), which leads to repression of target transcripts. In plants, a dsRNA precursor is cleaved by Dicer into small-scale dsRNA (dark-green box). In that location exist multiple amplification pathways; broadly, an RdRP can synthesise a complementary strand by elongating a small RNA bound to its target RNA. Found Dicer proteins tin then cleave this newly generated dsRNA. to produce many secondary siRNAs that tin can repress target transcripts via Ago, or begin another cycle of small RNA amplification
Similarly, product of minor RNAs was observed post-DNA damage in plants. The plant orthologs of Dicer protein, DCLs, are required for efficient DSB repair when A. thalina is challenged with IR.9 Utilising next-generation sequencing (NGS), it was shown that DNA damage-induced minor RNAs (diRNAs) arose in the proximity of the DSB sites. Interestingly, although these diRNAs are required for proficient repair, they are non involved in the initial recognition of DSBs indicated by the continued phosphorylation of Histone H2A.X.9
Recently, the importance of diRNAs in the DDR pathway was highlighted in metazoa. Several publications have documented the requirement for small RNAs, or sure components of the small RNA biogenesis machinery, in proficient DNA repair signalling.viii, 10, eleven, 13, 19 It is largely agreed that the key RNAse 3 family enzymes that process pocket-sized RNA precursors, Drosha and Dicer, have a role in the Deoxyribonucleic acid repair response.nine, ten, 11 Indeed, the loss of diRNAs or the small RNA biogenesis machinery, appears to affect the DNA repair process and have an impact on repair pathway choice.viii, 10, xvi Which stage inside the DDR pathway is affected by the loss of diRNAs and related proteins is currently contested (see Figure ane). Nevertheless, similarly to plants, it is thought that the initial phosphorylation of histone variant H2A.X is not affected past the loss of diRNAs.8, 11 It is likewise noteworthy that RNA polymerase Ii activity has been implicated in this procedure.xi
The generation of small RNA is a multistep biological process (Figure 2). Various accessory proteins such equally DGCR8 are required alongside Dicer and Drosha,twenty but their interest in Deoxyribonucleic acid repair has not been investigated in depth. Moreover, the participation of key downstream effectors in the RNAi pathway, namely the Argonautes, is also contested.8, ten, 11, 21 Currently, the mechanism past which the diRNAs direct influence repair upshot is nether all-encompassing investigation. Nevertheless, it has been reported that in Drosophila cells, these pocket-size RNAs can serve as endo-siRNAs to suppress existing transcripts arisen from the portion of DNA harbouring the DSB, every bit was proposed in Northward. crassa.13 Thus far, the evidence supporting the being of Deoxyribonucleic acid diRNAs has come from two main sources: deep sequencing and the isolation of the small-scale RNA fraction for use in rescue experiments, which volition be discussed in detail in this review.
Search for the I: Using NGS to Place diRNAs
How can the generation of small RNA in a Deoxyribonucleic acid damage-specific context be detected? Ordinarily used external DNA damage agents, such every bit IR, lead to the generation of multiple breaks at random genomic sites. This makes the task of discovery of novel RNA species with the use of an NGS approach well-nigh impossible, as breaks demand to occur in known defined sequences for this experimental strategy to work. To date, three studies have used brake enzyme-based systems in animal cells combined with NGS to detect diRNAs, which map to the vicinity of the cutting site.9, xi, 13 These reports relied on the ectopic expression of rare restriction enzymes targeted to specific pre-integrated loci in the genome. Two different systems were adopted in human cell lines: the DR-GFP Hr reporter or a Lac-/Tet-operator repeat-flanking reporter,22, 23 both of which comprise a unmarried recognition site for the uniquely cutting meganuclease I-SceI (Figures 3a and b, see also Figure 4 and the more in-depth word of the DR-GFP reporter assay below). Following the transfection and expression of I-SceI for 12 to 24 h, small RNAs were sequenced past NGS. These two studies reported the existence of small RNAs around the break sites.ix, 11 Even so, the verbal roles of these small RNAs are yet to exist determined.
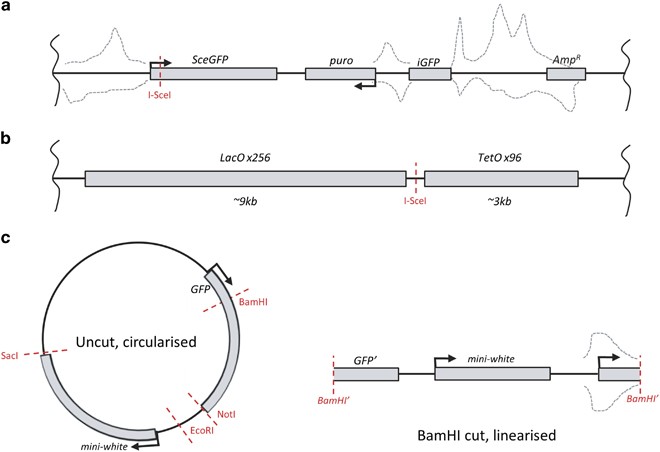
Schematics of reporter systems used for small-scale RNA sequencing experiments. (a) Wei et al. ix DR-GFP genomically integrated reporter. Transfection of I-SceI results in cleavage near the transcriptional start site (TSS) of GFP. Small RNA was sequenced and mapped back to the reporter sequence. (b) Francia et al. 11 genomically integrated Lac-/Tet-operator-flanked I-SceI site. This reporter lacks transcriptional activity but is highly repetitive. Small RNA was detected later on I-SceI transfection but at low levels (47 full reads after transfection versus 20 reads without). No data on where these RNAs mapped to was provided. (c) Michalik et al. thirteen Drosophila expression plasmids, either circularised or linearised. Here just the BamHowdy linearised vector is shown as it produced the highest number of pocket-sized RNA reads. Grey dashed lines correspond the approximate distribution of pocket-sized RNA mapping dorsum to the locus, where positional data was supplied in the manuscript. Right-angled arrows correspond TSS, whereas vertical wavy line denotes integration within the genome
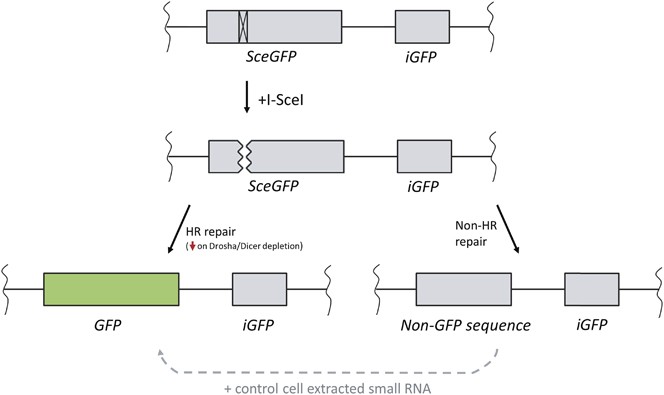
Detailed schematic of the DR-GFP Hr repair reporter as in Figure 3a, used for RNA rescue experiments. A copy of the reporter is integrated into the genome to provide advisable chromatin context. Insertion of an I-SceI restriction site within the GFP ORF results in a nonsense product that will produce no green fluorescence. Later on the induction of I-SceI cleavage, the cell can repair the resulting DSB via HR using the downstream internal GFP sequence (iGFP), producing a full-length GFP product. If HR is impaired, the break will instead be repaired via an alternating pathway, such as NHEJ, resulting in a sequence lacking full GFP coding region. The extent of deletion is dependent upon the not-HR mechanism called by the cell, but any loss of sequence within the I-SceI restriction site will prevent any further cutting. In the experiments by Wei et al. ix and Wang and Goldstein,10 the loss of Drosha and Dicer resulted in a lack of GFP indicating a deficiency in HR repair; however, when small RNAs extracted from command cells were incubated with these deficient cells for 1 h, GFP was found to be expressed (denoted by dashed arrow)
An culling approach has involved the transfection of either round (uncut) or linearised (cut) plasmids into Drosophila S2 cells (Effigy 3c).23 Similarly, information technology was found that small RNA tin can be generated from the vicinity of Deoxyribonucleic acid suspension sites. Strikingly, these modest RNAs can exist generated in response to either blunt or staggered DNA ends and they proceed to serve every bit endo-siRNAs to repress corresponding transcripts in trans.thirteen It is noteworthy that this response tin can only be provoked by a Deoxyribonucleic acid DSB, but non a nicked DNA.thirteen A recent follow-up article by the same group utilised a similar GFP-based reporter to that previously described13 and over again reported pocket-sized RNAs mapping to the damaged locus following damage induction.2, 13, 24 In summary, these reports by Forstemann and co-workers advise that diRNA function in Drosophila appears to be more similar to plant-based qiRNAs, which remove aberrant transcripts or aid in other RNA metabolic processes.13, xiv, 24 In contrast, in mammalian cells, they are reported to have a direct contribution to the Dna repair processes.x, 11
Although in that location are many differences between the several studies published thus far, it is important to note that all the groups take found an enrichment of small RNAs mapping to exogenous loci following Deoxyribonucleic acid damage. Information technology is articulate from these studies that the miRNA biogenesis enzymes and RNA species are critically involved in the DNA repair process with multiple groups presenting similar observations in a diverse collection of experimental settings. Here, nosotros discuss these results and examine the merit of the unlike approaches taken, integrating them to develop new hypotheses for how RNA could participate in Deoxyribonucleic acid repair.
Needles in a Haystack: Methodologies and Difficulties of diRNA Discovery past NGS
The advent of NGS has revolutionised the RNA field, allowing robust quantitation of RNA changes between samples and discovery of novel transcripts and splice variants in a loftier-throughput fashion. As such, NGS was the sensible selection for discovering novel RNA species at DSBs.
These small RNAs were commencement identified by NGS in plants and humans.9, 11 Reads mapping to regions proximal to the integration locus of the Hr repair reporter were observed, with the earliest peaks of RNA reads visualised 12 h following the appearance of DSB induced past the transfection of I-SceI (Figure 3a).25 Interestingly, the bulk of these mapped RNAs practise not appear to accept arisen directly from the DNA intermission site. Instead, they were mapped to the sequence upstream of the start site of the cut GFP gene, located upstream of the puromycin resistance gene, or downstream of the homologous GFP sequence.ix Information technology thus appears that the minor RNAs are mapped to highly transcribed regions proximal to the break site, making information technology unclear as to whether they are true de novo transcripts, or degradation products of pre-existing mRNAs. Small RNAs were also shown to be generated in plants mail service-DNA damage, with read numbers considerably higher than those in human being cells.ix One attribute of plant biological science that may contribute to this difference is the existence of RdRPs (Effigy 2, green box). It is possible that subsequently DNA damage, RdRPs may be activated to amplify nascent transcripts into dsRNAs that are then further processed into diRNAs by Dicer, in a similar manner to that reported in N. crassa. Interestingly, the authors showed that RNA politician Four, which is responsible for transcription from repetitive and transposable elements, was critical for the production of diRNAs in plants and loss of this enzyme significantly reduced repair efficiency.9 In plants, RDR2 is the RdRP responsible for amplifying pol Four transcripts to produce the hc-siRNA class of small RNAs.26, 27 When RDR2 was ablated, the number of diRNAs was hugely reduced, however, repair efficiency was unaffected.9 This discrepancy between overall small RNA levels and repair efficiency suggests that the nascent RNAs produced post-obit damage are important for repair resolution, but perhaps the amplification of secondary RNA products past RDR2 is non. This could stand for a distinction in the role of small RNAs betwixt plants and metazoa, where in animals an amplification loop is non required for a secondary part for functional RNA molecules. Alternatively, a like mechanism may be utilising a yet undiscovered RdRP activeness in animals. For example, in humans TERT-RMRP and RNA polymerase Two have been demonstrated to accept slight RdRP action.28, 29, thirty
As these reporter systems are nether the control of viral promoters with high basal activity, it is entirely plausible that the pre-existing, highly abundant long RNAs transcribed from reporter loci are degraded as role of the DDR.31 The approach taken by Francia et al. xi partially addresses this possibility: the Tet-/Lac-flanked I-SceI sequence used is devoid of any transcriptional elements and thus any new RNAs should exist generated in a Dna damage-dependent style (see Effigy 3b). Post-obit deep sequencing, they reported a full of 47 reads arising from the 12 kb integrated locus when cut with the endonuclease, compared with xx in uncut controls. As the parental cell lines produced no pocket-sized RNAs that mapped to this sequence, these small RNAs are indeed sequence-specific and dependent on reporter integration.11 Yet, with the low read counts, and modest enrichment to a higher place background level, it is difficult to convincingly conclude that new RNA species are specifically transcribed post-damage. Information technology should also exist noted that the presence of the 20 pocket-size RNA reads in the absenteeism of harm suggests these RNAs may non be entirely damage dependent.
When similar deep sequencing investigations were performed in cells depleted of Drosha and Dicer, the analyses revealed that but loss of Dicer reduced small RNA counts significantly.11 The lack of a role for Drosha in production of the small RNAs simply the requirement for Dicer suggests that diRNAs may be produced from the cleavage of a longer dsRNA forerunner rather than from any pri-miRNA-like secondary structures within an ssRNA precursor (see also Effigy 5 office a and b). It is of import to remember that Drosha has been observed to impact DNA repair efficiency.11 This may echo the previously discussed ascertainment in plants that generation of the small RNAs (by politico IV and RDR2) was unconnected to repair issue. Information technology should as well exist noted that Dicer and Drosha are known to have a role in not-canonical termination of new RNA transcripts.19, 32, 33 Whether this activeness of Drosha is utilised after DNA impairment warrants further investigation.
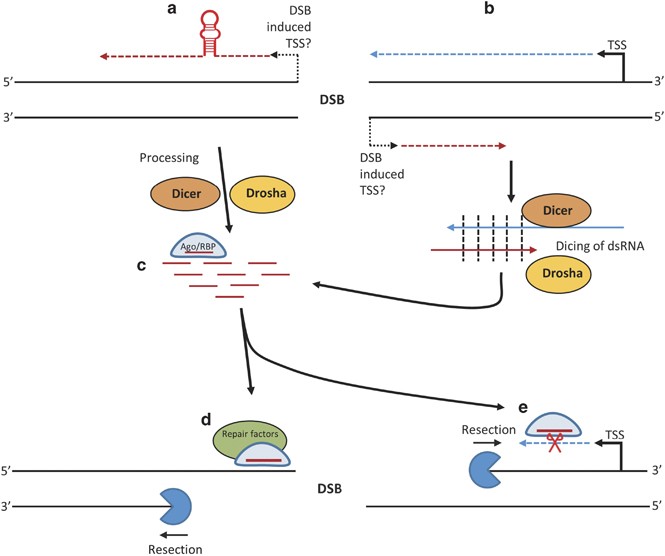
Postulated biogenesis of diRNAs and their function in DDR. Directionality of transcription, transcripts, and resection is 5' to 3', denoted by arrows. (a) A new transcript is formed from the break site. Any secondary structure formed past this transcript that can be recognised by Drosha and Dicer is candy into pocket-size RNAs, mirroring miRNA generation. (b) Where a cut occurs within an actively transcribing cistron, a new cease-dependent transcription event could take identify resulting in formation of an antisense RNA. These RNA species tin can amalgamate to form dsRNA, and are then diced into small-scale RNA substrates by Dicer. (c) The resulting small RNAs could then exist incorporated into an Argonaute or another RBP, to carry out diRNA functions, which are hypothesised to be: (d) recruitment of repair and chromatin remodelling factors to the site of impairment, (e) degradation of potentially aberrant transcripts or other as-of-all the same unexplored functions
Using a insufficiently straightforward system, Michalik et al. xiii transfected Drosophila S2 cells with several exogenous sequences: a GFP expression vector and an unrelated yeast plasmid, which were either linearised ('cut') or circularised ('uncut') (see Effigy 3c). A small linear PCR amplicon comprising firefly luciferase coding sequence was also used equally an boosted control. Following deep sequencing, small RNAs were mapped to the vector sequences with significantly more reads arising from the linearised Drosophila vector than the circularised one. Depending on the brake enzyme used to generate the linearised plasmids, different patterns of small-scale RNA were produced. This suggests the context of the DSB may affect the pattern of newly transcribed RNA. The small RNAs appeared to map predominantly upstream of the cut site, with the bulk of small RNAs arising from regions adjacent to the GFP promoter. Similar to the studies conducted by Francia et al. 11 and Wei et al.,9 these data practise not distinguish between nonspecific deposition of RNA produced from the reporter as a outcome of recognition of DSB-similar structures, or a deliberate processing upshot that generates pocket-sized RNAs that may have a directly mechanistic role in Deoxyribonucleic acid repair.xiii Intriguingly, the lack of reads mapping to the PCR product, but a surprisingly high number of reads for the command yeast plasmid, suggests that some potential promoter activity may be required. Ane may argue that a possible pitfall of this plasmid-based approach is that the jail cell is exposed to Deoxyribonucleic acid lacking any chromatin construction. Thus, it is hard to relate these observations to Deoxyribonucleic acid damage inside a genomic context. Too, it is possible that these sequences are generated in response to introduction of foreign genetic textile by an anti-viral or retrotransposon defence force machinery contained of the DDR.34, 35, 36 However, the lack of a response from the transfected control PCR product suggests this is not the case.
Considering all these limitations, nosotros propose that the ideal experimental setting to investigate the existence of diRNAs requires a system that produces DSBs at a range of different sites within the genome. This style, the potential interest of chromatin construction or transcriptional status can be investigated. Thus far, ii endogenous brake enzyme-based systems have been extensively utilised in the DDR field: AsiSI3, 37, 38 and I-PpoI.x, 39 Alternatively, the CRISPR-Cas9 system also allows the induction of DSBs at specific sites of the genome:xl, 41 using specially designed guide RNAs, the dynamics of diRNA production could be investigated fifty-fifty further by comparing DSBs generated proximal with promoters and transcriptional start sites to those generated further abroad within the aforementioned gene. Such approaches could help elucidate whether newly produced RNAs arise from transcription events at the DNA intermission site, or from a promoter or cryptic promoter in the vicinity of the DSBs.
To Mend a Broken Heart: Pre-isolated Small-scale RNA Fraction Acts in Deoxyribonucleic acid Repair
The major culling strategy used to investigate the existence of small RNAs produced following Dna damage involved the isolation of the pocket-size RNA from cells and delivery of that RNA into cells that lack the power to produce them. Cell lines conveying a DR-GFP integrated reporter (Effigy 4) were incubated with a pre-extracted modest RNA fraction from damaged or undamaged cells.nine, 11 This reporter consists of a GFP open reading frame containing an inserted I-SceI recognition site, which when transcribed results in an abnormal transcript. I-SceI induction leads to cleavage at the non-functional GFP, and allows repair via Hr using the downstream intact sequence every bit template. This results in the cosmos of a copy of GFP that tin can express a full-length protein. The consequence of HR repair tin can so exist measured past analysing GFP-positive cells by period cytometry.
Depletion of Drosha or Dicer in cells carrying this transgene following 2 days of damage resulted in a reduction in GFP-positive cells indicating that HR was impaired.9, x Interestingly, two groups reported that incubation with modest RNAs isolated from previously damaged cells for just 1h could restore HR efficiency.8, 10 In contrast, modest RNAs extracted from undamaged cells failed to accomplish such rescue.x This suggests the interest of an RNA species with sequence-specific characteristics in the DDR process. The processes of transcription through to translation of a gene can take from minutes to hours, while the process of maturation and folding of fluorescent proteins may take even longer.42, 43 Therefore, it is unexpected that the incubation of pocket-sized RNAs for merely an hour, days later the induction of DNA damage at GFP loci, can rescue expression of GFP inside such a curt timeframe, peculiarly when HR repair only occurs during the S/G2-phase of the jail cell cycle. Moreover, given the nature of these experiments, if repair of cut sites is carried out by an fault-prone non-Hour mechanism, mutations volition be introduced into the cut site preventing subsequent cleavage events (see Figure 4). Therefore, although information technology is possible for this to occur, it is unclear whether incubation with modest RNAs 1 h before FACS analysis can result in restoration of functional HR repair at this specific break site.
Nevertheless, it is important to remember that this method was not the simply approach used past the authors. Small RNAs were isolated from damaged cells and were able to restore 53BP1 DDR foci in cells pre-treated with RNase A.11
Last Remarks and Future Perspectives
Recent advances in deep sequencing have made information technology possible to conduct refined experiments leading to the proposition of involvement of human small RNA processing machinery in DDR. This is peculiarly interesting given certain reports demonstrating that RNA molecules may be used as templates for Deoxyribonucleic acid repair in yeast.12 Although it is largely agreed that Dicer and Drosha have some part in DNA repair, its machinery is still elusive (see Figure 5).8, 9, 10, 11 Likewise, it is not clear whether this machinery involves the typical co-factors of Drosha and Dicer, such equally DGCR8, DDX5, DDX17 and TRBP. Given that Dicer and Drosha are involved in the not-canonical termination of transcription and modulation of RNA polymerase II activeness, it is possible that sure interaction partners may not exist required for the Deoxyribonucleic acid repair-related activeness.xix, 32, 33
1 primary direction for further investigation is the identity and biogenesis mechanism of the RNA species involved in DNA repair: whether these species are bona fide new small RNA transcripts derived from the vicinity of the break site, or degradation products of pre-existing transcripts. Whether they tin serve as RNA templates (or remnants of RNA templates) that actively participate in the repair procedure is unknown. However, it should exist noted that while several classes of non-coding RNA are produced in association with Dna damage, the impact of these RNAs on the repair process in different model systems is varied (meet Table one). For example, found-based aRNAs, qiRNAs and hc-siRNA require RdRP activity, and they have been shown to induce the degradation of transcripts.14, 18, 26 Similarly, diRNAs are reported to serve as endo-siRNAs in Droshophila systems.thirteen, 24 All the same, the small RNA produced in mammalian cells, termed diRNAs, are reported to be directly involved in the repair procedure, but the mechanism of action is even so nether debate.viii, nine, 10, 11
Recent studies have provided the first direct evidence for an RNA-templated repair machinery in both yeast and human being cells, the latter of which curiously utilises the NHEJ machinery.12, 44 Again in yeast, an fifty-fifty more than recent paper also demonstrates the germination of RNA:Deoxyribonucleic acid hybrids at sites of Dna harm, showing a stiff link between transcription and Dna repair.45 Alternatively, they may besides be involved in the process of modulating chromatin states, in a manner similar to piwi-interacting RNA in germ cells.46 Provided that these small RNAs function in a sequence-specific manner analogous to RNAi, ane should expect that an Argonaute-like protein would exist required to facilitate scanning and base of operations pairing with its genomic target (Effigy five).47 Currently, the jury is still out regarding the verbal function of Ago2 protein in DDR.eight, x, 21
With recent reports documenting crosstalk between the Deoxyribonucleic acid repair processes and RNA transcription, processing and splicing mechanism, one can only envisage an even more intertwined interaction betwixt RNA and the DNA repair procedure.3, 25, 48
References
-
Ciccia A, Elledge SJ . The Dna damage response: making it safe to play with knives. Mol Cell 2022; 40: 179–204.
-
Chapman JR, Taylor MR, Boulton SJ . Playing the end game: Dna double-strand break repair pathway selection. Mol Cell 2022; 47: 497–510.
-
Aymard F, Bugler B, Schmidt CK, Guillou E, Caron P, Briois S et al. Transcriptionally agile chromatin recruits homologous recombination at DNA double-strand breaks. Nat Struct Mol Biol 2022; 21: 366–U172.
-
Lu WT, Lemonidis One thousand, Drayton RM, Nouspikel T . The Fanconi anemia pathway is downregulated upon macrophage differentiation through two singled-out mechanisms. Jail cell Cycle 2022; ten: 3300–3310.
-
Unno J, Itaya A, Taoka M, Sato One thousand, Tomida J, Sakai W et al. FANCD2 binds CtIP and regulates Deoxyribonucleic acid-finish resection during Dna interstrand crosslink repair. Cell Rep 2022; vii: 1039–1047.
-
Panier S, Boulton SJ . Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol 2022; fifteen: 7–18.
-
Zimmermann M, de Lange T . 53BP1: pro choice in DNA repair. Trends Cell Biol 2022; 24: 108–117.
-
Gao M, Wei W, Li MM, Wu YS, Ba Z, Jin KX et al. Ago2 facilitates Rad51 recruitment and DNA double-strand break repair by homologous recombination. Cell Res 2022; 24: 532–541.
-
Wei W, Ba ZQ, Gao K, Wu Y, Ma YT, Amiard S et al. A office for pocket-sized RNAs in DNA double-strand break repair. Jail cell 2022; 149: 101–112.
-
Wang Q, Goldstein M . Small-scale RNAs recruit chromatin-modifying enzymes MMSET and Tip60 to reconfigure damaged Deoxyribonucleic acid upon double-strand break and facilitate repair. Cancer Res 2022; 76: 1904–1915.
-
Francia S, Michelini F, Saxena A, Tang D, de Hoon K, Anelli Five et al. Site-specific DICER and DROSHA RNA products command the Dna-impairment response. Nature 2022; 488: 231–235.
-
Keskin H, Shen Y, Huang F, Patel Thou, Yang T, Ashley K et al. Transcript-RNA-templated Deoxyribonucleic acid recombination and repair. Nature 2022; 515: 436–439.
-
Michalik KM, Bottcher R, Forstemann K . A small RNA response at Deoxyribonucleic acid ends in Drosophila. Nucleic Acids Res 2022; 40: 9596–9603.
-
Lee HC, Chang SS, Choudhary S, Aalto AP, Maiti 1000, Bamford DH et al. qiRNA is a new blazon of minor interfering RNA induced by DNA harm. Nature 2009; 459: 274–277.
-
Hall IM, Noma M, Grewal SI . RNA interference machinery regulates chromosome dynamics during mitosis and meiosis in fission yeast. Proc Natl Acad Sci USA 2003; 100: 193–198.
-
Storici F, Bebenek K, Kunkel TA, Gordenin DA, Resnick MA . RNA-templated DNA repair. Nature 2007; 447: 338–341.
-
Ghildiyal M, Zamore PD . Small silencing RNAs: an expanding universe. Nat Rev Genet 2009; 10: 94–108.
-
Lee HC, Aalto AP, Yang Q, Chang SS, Huang One thousand, Fisher D et al. The Deoxyribonucleic acid/RNA-dependent RNA polymerase QDE-one generates aberrant RNA and dsRNA for RNAi in a process requiring replication protein A and a DNA helicase. PLoS Biol 2022; viii: x.
-
Castel SE, Ren J, Bhattacharjee S, Chang AY, Sanchez M, Valbuena A et al. Dicer promotes transcription termination at sites of replication stress to maintain genome stability. Cell 2022; 159: 572–583.
-
Finnegan EF, Pasquinelli AE . MicroRNA biogenesis: regulating the regulators. Crit Rev Biochem Mol Biol 2022; 48: 51–68.
-
Gioia U, d'Adda di Fagagna F . Human nuclear ARGONAUTE 2 interacts in vivo only with pocket-size RNAs and non with DNA. Cell Bicycle 2022; xiv: 2001–2002.
-
Soutoglou Due east, Dorn JF, Sengupta G, Jasin M, Nussenzweig A, Ried T et al. Positional stability of single double-strand breaks in mammalian cells. Nat Jail cell Biol 2007; 9: 675–U121.
-
Pierce AJ, Johnson RD, Thompson LH, Jasin M . XRCC3 promotes homology-directed repair of Deoxyribonucleic acid damage in mammalian cells. Gene Dev 1999; 13: 2633–2638.
-
Schmidts I, Bottcher R, Mirkovic-Hosle Thou, Forstemann Grand . Homology directed repair is unaffected past the absence of siRNAs in Drosophila melanogaster. Nucleic Acids Res 2022; 44: 8261–8271.
-
Beli P, Lukashchuk North, Wagner SA, Weinert BT, Olsen JV, Baskcomb Fifty et al. Proteomic investigations reveal a part for RNA processing factor THRAP3 in the Deoxyribonucleic acid harm response. Mol Jail cell 2022; 46: 212–225.
-
Haag JR, Ream TS, Marasco Chiliad, Nicora CD, Norbeck Advertizement, Pasa-Tolic L et al. In vitro transcription activities of Pol 4, Politico Five, and RDR2 reveal coupling of Pol Iv and RDR2 for dsRNA synthesis in institute RNA silencing. Mol Prison cell 2022; 48: 811–818.
-
Chapman EJ, Carrington JC . Specialization and development of endogenous small-scale RNA pathways. Nat Rev Genet 2007; 8: 884–896.
-
Maida Y, Yasukawa Thou, Furuuchi M, Lassmann T, Possemato R, Okamoto N et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature 2009; 461: 230–U104.
-
Lehmann E, Brueckner F, Cramer P . Molecular basis of RNA-dependent RNA polymerase II activity. Nature 2007; 450: 445–449.
-
Wagner SD, Yakovchuk P, Gilman B, Ponicsan SL, Drullinger LF, Kugel JF et al. RNA polymerase II acts as an RNA-dependent RNA polymerase to extend and destabilize a non-coding RNA. EMBO J 2022; 32: 781–790.
-
Thomas MP, Liu X, Whangbo J, McCrossan G, Sanborn KB, Basar E et al. Apoptosis triggers specific, rapid, and global mRNA decay with 3' uridylated intermediates degraded past DIS3L2. Cell Rep 2022; eleven: 1079–1089.
-
Dhir A, Dhir Southward, Proudfoot NJ, Jopling CL . Microprocessor mediates transcriptional termination of long noncoding RNA transcripts hosting microRNAs. Nat Struct Mol Biol 2022; 22: 319.
-
Gromak N, Dienstbier Thou, Macias S, Plass M, Eyras E, Caceres JF et al. Drosha regulates gene expression independently of RNA cleavage function. Cell Rep 2022; 5: 1499–1510.
-
Soifer HS, Zaragoza A, Peyvan M, Behlke MA, Rossi JJ . A potential function for RNA interference in decision-making the activity of the human being LINE-1 retrotransposon. Nucleic Acids Res 2005; 33: 846–856.
-
Yang N, Kazazian HH Jr . L1 retrotransposition is suppressed by endogenously encoded small interfering RNAs in homo cultured cells. Nat Struct Mol Biol 2006; thirteen: 763–771.
-
Campo South, Gilbert KB, Carrington JC . Pocket-size RNA-based antiviral defense force in the phytopathogenic fungus Colletotrichum higginsianum. PLoS Pathogens 2022; 12: e1005640.
-
Iacovoni JS, Caron P, Lassadi I, Nicolas E, Massip L, Trouche D et al. High-resolution profiling of gamma H2AX around DNA double strand breaks in the mammalian genome. EMBO J 2022; 29: 1446–1457.
-
Massip L, Caron P, Iacovoni JS, Trouche D, Legube Yard . Deciphering the chromatin landscape induced effectually Dna double strand breaks. Cell Bicycle 2022; 9: 2963–2972.
-
Berkovich E, Monnat RJ, Kastan MB . Roles of ATM and NBS1 in chromatin structure modulation and Deoxyribonucleic acid double-strand break repair. Nat Cell Biol 2007; 9: 683–U137.
-
Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J . RNA-programmed genome editing in human cells. eLife 2022; 2: e00471.
-
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE et al. RNA-guided man genome engineering via Cas9. Science 2022; 339: 823–826.
-
Jonkers I, Kwak H, Lis JT . Genome-wide dynamics of Pol II elongation and its coaction with promoter proximal pausing, chromatin, and exons. eLife 2022; 3: e02407.
-
Iizuka R, Yamagishi-Shirasaki M, Funatsu T . Kinetic study of de novo chromophore maturation of fluorescent proteins. Anal Biochem 2022; 414: 173–178.
-
Chakraborty A, Tapryal N, Venkova T, Horikoshi N, Pandita RK, Sarker AH et al. Classical non-homologous end-joining pathway utilizes nascent RNA for fault-free double-strand break repair of transcribed genes. Nat Commun 2022; vii: 13049.
-
Ohle C, Tesorero R, Schermann G, Dobrev N, Sinning I, Fischer T . Transient RNA-Deoxyribonucleic acid hybrids are required for efficient double-strand break repair. Cell 2022; 167: 1001–1013 e1007.
-
Siomi MC, Sato M, Pezic D, Aravin AA . PIWI-interacting pocket-sized RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol 2022; 12: 246–258.
-
Salomon We, Jolly SM, Moore MJ, Zamore PD, Serebrov 5 . Single-molecule imaging reveals that argonaute reshapes the binding backdrop of its nucleic acid guides. Jail cell 2022; 162: 84–95.
-
Barbarous KI, Gorski JJ, Barros EM, Irwin GW, Manti L, Powell AJ et al. Identification of a BRCA1-mRNA splicing complex required for efficient Deoxyribonucleic acid repair and maintenance of genomic stability. Mol Cell 2022; 54: 445–459.
Acknowledgements
The authors are funded by the Medical Research Council (MRC), United kingdom. AW is supported by the BBSRC and W-TL was partly supported by MRC Centenary Award. We thank Gaelle Legube (CNRS, Academy of Toulouse Three) for critical reading of the manuscript.
Author data
Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Blagosklonny
Rights and permissions
This piece of work is licensed under a Creative Commons Attribution 4.0 International License. The images or other 3rd party material in this article are included in the article'south Creative Commons license, unless indicated otherwise in the credit line; if the fabric is not included under the Creative Eatables license, users volition need to obtain permission from the license holder to reproduce the textile. To view a re-create of this license, visit http://creativecommons.org/licenses/by/four.0/
Reprints and Permissions
About this commodity
Cite this commodity
Hawley, B., Lu, WT., Wilczynska, A. et al. The emerging function of RNAs in Deoxyribonucleic acid damage repair. Cell Decease Differ 24, 580–587 (2017). https://doi.org/10.1038/cdd.2017.sixteen
-
Received:
-
Revised:
-
Accepted:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1038/cdd.2017.xvi
Farther reading
Source: https://www.nature.com/articles/cdd201716
Posted by: norrisaffeas.blogspot.com


0 Response to "What Can Be Used To Repair Mrna"
Post a Comment